Recursion Provides Business Updates and Reports Third Quarter 2023 Financial Results
- Entered into a collaboration with
Tempus giving Recursion access to over 20 petabytes of multimodal oncology data for the purpose of training causal AI models together with Recursion’s proprietary data and for designing biomarker and patient stratification strategies for clinical programs - Expansion of Recursion’s in-house supercomputer, BioHive-1, with NVIDIA H100 GPUs will likely make it the most powerful computer wholly owned or operated by any biopharma company and among the 50 most powerful supercomputers on the Top500 List
- Updated collaboration with Bayer to deliver against their thematic focus in precision oncology with higher per program milestones and broader use of Recursion’s OS
“Since our founding we have led TechBio with a belief that the next generation of biopharma leaders would operate at the intersection of scaled datasets and accelerated computing,” said
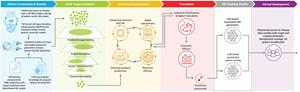
Recursion OS: Industrializing drug discovery to transform BioTech into TechBio
Summary of Business Highlights
- Platform
- Tempus Collaboration
- Oncology-Focused, Precision Medicine Data:
Tempus has built one of the world’s largest oncology-focused clinical and DNA/RNA molecular observational datasets. Our new collaboration withTempus gives Recursion preferred access to these data. When combined with Recursion’s proprietary dataset of over 25 petabytes of interventional biological and chemical data, Recursion will now have approximately 50 petabytes of proprietary data fit for the purpose of machine learning at its disposal, enabling us to improve the training of causal AI/ML models of biology. When applied to our genome-wide reverse genetics platform, these data could facilitate the discovery of novel associations and mechanisms not otherwise identifiable in the clinical and forward genetics data fromTempus . Additionally, this patient-linked data will be used to support translating innovative therapeutics from Recursion’s platform directly to patients using novel biomarker and patient stratification strategies. - Terms of the Tempus Collaboration: Recursion entered into an agreement with
Tempus to access its patient-centric data as part of a 5-year licensing agreement. Recursion will make annual payments toTempus in cash or equity ranging between$22M and$42M each year, up to$160M in aggregate, over the next 5 years in exchange for continued and updated data access and use rights for therapeutic development purposes.
- Oncology-Focused, Precision Medicine Data:
- Supercomputer Expansion: We have committed to working with NVIDIA to expand BioHive-1, our on-premise supercomputer. After the expansion, which will be completed in the first half of 2024, BioHive-1 computational capacity will increase by over 4x (adding more than 500 NVIDIA H100 GPUs to the more than 300 NVIDIA A100s already in place). We project that upon completion and benchmarking, BioHive-1 will be in the top 50 most powerful supercomputers in the world across any industry (according to the Top500 list) and will be the most powerful supercomputer owned and operated by any biopharma company. These additional computational resources will continue to support the construction of the largest foundation models across biology and chemistry using Recursion’s vast datasets and data generation capabilities as well as tools based on interactive large language models and autonomous agents.
- Foundation Models: Our supercomputer expansion is meant to build on the deployment of our first Phenomics Foundation Model, PHENOM-1, which is a vision transformer utilizing hundreds of millions of parameters trained on billions of biological images from our proprietary phenomics library. PHENOM-1 demonstrated the scaling hypothesis within a biological context, namely that larger models trained on more diverse datasets lead to increased performance and emergent properties. With our recent acquisitions of digital chemistry company Cyclica and the deep-learning research team at Valence Discovery (now
Valence Labs ), the vast patient-centric data fromTempus and our own growing proprietary multi-omic datasets, we anticipate the construction and application of more foundation models and large language models across biology, chemistry and translation. Together, we believe these increasingly sophisticated models will enable us to drive new, better programs into clinical development both in our own pipeline and with our current and future partners at scale.
- Tempus Collaboration
- Pipeline
- Cerebral Cavernous Malformation (CCM) (REC-994): Our Phase 2 SYCAMORE clinical trial is a double-blind, placebo-controlled safety, tolerability and exploratory efficacy study of this drug candidate in participants with CCM. This study was fully enrolled as of
June 2023 with 62 participants and the vast majority of participants who have thus far finished their first year of treatment have enrolled in the long-term extension study. We expect to share Phase 2 proof-of-concept data in H2 2024. - Neurofibromatosis Type 2 (NF2) (REC-2282): Our Phase 2/3 POPLAR clinical trial is a two part study of REC-2282 in participants with progressive NF2-mutated meningiomas due either to syndromic disease or initiating mutations in the meningiomas. Part 1 of the study is ongoing and is exploring two doses of REC-2282 in approximately 23 adults and 9 adolescents. We expect to share Phase 2 safety, tolerability, pharmacokinetics, and preliminary efficacy in H2 2024.
- Familial Adenomatous Polyposis (FAP) (REC-4881): Our Phase 2 TUPELO clinical trial is a two part study of REC-4881 in participants with FAP. Evaluation of three dose levels is ongoing, thereafter a dose expansion phase will commence evaluating the recommended Phase 2 dose in approximately 30 participants. We expect to share Phase 2 safety, tolerability, pharmacokinetics, and preliminary efficacy in H1 2025.
- AXIN1 or APC Mutant Cancers (REC-4881): Our Phase 2 LILAC clinical trial is a biomarker enriched two part study of REC-4881 in participants with unresectable, locally advanced or metastatic cancer with AXIN1 or APC mutations. The study will initiate in late Q4 2023 or early Q1 2024 and will explore the safety and efficacy of REC-4881 across three dose levels in 30-40 participants.
- Clostridioides difficile Infection (REC-3964): In early
September 2023 , we announced completion of our Phase 1 clinical trial and reported that REC-3964 had been well tolerated in healthy volunteers with no serious adverse events. We expect to initiate a Phase 2 proof-of-concept study in patients with recurrent Clostridioides difficile infection in 2024. - RBM39 HR-Proficient Ovarian Cancer: RBM39 is a novel CDK12-adjacent target identified by the Recursion OS. We believe we can modulate this target to produce a therapeutic effect in HR-proficient ovarian cancer and potentially in other tumor types. This program is in the preclinical stage and IND-enabling studies are progressing.
- Cerebral Cavernous Malformation (CCM) (REC-994): Our Phase 2 SYCAMORE clinical trial is a double-blind, placebo-controlled safety, tolerability and exploratory efficacy study of this drug candidate in participants with CCM. This study was fully enrolled as of
- Partnerships
- Bayer: Bayer and Recursion have signed an update to their collaboration around a select set of oncology programs. This decision allows Bayer to leverage Recursion’s capabilities to identify novel targets and compounds applicable to traditionally undruggable oncology indications as well as Recursion’s access to expansive oncology-focused, patient-centric data from
Tempus for their closely partnered programs. Under the amended and restated agreement, Bayer will pay Recursion increased per program milestones which may be up to$1.5B for up to 7 oncology programs as well as royalties on net sales. In this oncology-focused collaboration, Recursion will use many of the new tools it has developed since the collaboration was first signed to potentially identify and nominate programs rapidly. - Roche-
Genentech : InOctober 2023 , Recursion announced that Roche-Genentech optioned its first partnership program in GI-oncology. This milestone represents a critical step in our joint efforts to initiate and advance new therapeutic programs using Recursion’s approach to map and navigate biology and chemistry. In the near-term, there is the potential for option exercises associated with map building or data sharing initiatives as well as option exercises associated with additional partnership programs.
- Bayer: Bayer and Recursion have signed an update to their collaboration around a select set of oncology programs. This decision allows Bayer to leverage Recursion’s capabilities to identify novel targets and compounds applicable to traditionally undruggable oncology indications as well as Recursion’s access to expansive oncology-focused, patient-centric data from
Third Quarter 2023 Financial Results
- Cash Position: Cash and cash equivalents were
$387.3 million as ofSeptember 30, 2023 . This cash position excludes the$3 million to be paid by Roche-Genentech for optioning its first partnership program in GI-oncology. - Revenue: Total revenue was
$10.5 million for the third quarter of 2023, compared to$13.2 million for the third quarter of 2022. The decrease was due to the timing of workflows from our strategic partnership with Roche-Genentech.. - Research and Development Expenses: Research and development expenses were
$70.0 million for the third quarter of 2023, compared to$40.8 million for the third quarter of 2022. The increase in research and development expenses was due to increased platform costs as we have expanded and upgraded our capabilities. - General and Administrative Expenses: General and administrative expenses were
$29.2 million for the third quarter of 2023, compared to$19.5 million for the third quarter of 2022. The increase in general and administrative expenses was due to an increase in salaries and wages of$5.8 million and increases in software and depreciation expenses. - Net Loss: Net loss was
$93.0 million for the third quarter of 2023, compared to a net loss of$60.4 million for the third quarter of 2022. Net Cash : Net cash used in operating activities was$72.9 million for the third quarter of 2023, compared to net cash used in operating activities of$54.5 million for the third quarter of 2022.
About Recursion
Recursion is a clinical stage TechBio company leading the space by decoding biology to industrialize drug discovery. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously expands one of the world’s largest proprietary biological and chemical datasets. Recursion leverages sophisticated machine-learning algorithms to distill from its dataset a collection of trillions of searchable relationships across biology and chemistry unconstrained by human bias. By commanding massive experimental scale — up to millions of wet lab experiments weekly — and massive computational scale — owning and operating one of the most powerful supercomputers in the world, Recursion is uniting technology, biology and chemistry to advance the future of medicine.
Recursion is headquartered in
Media Contact
Media@Recursion.com
Investor Contact
Investor@Recursion.com
Consolidated Statements of Operations
| Condensed Consolidated Statements of Operations (unaudited) | ||||||||||||||||
| (in thousands, except share and per share amounts) | ||||||||||||||||
| Three months ended | Nine months ended | |||||||||||||||
| Revenue | 2023 | 2022 | 2023 | 2022 | ||||||||||||
| Operating revenue | $ | 10,102 | $ | 13,053 | $ | 33,252 | $ | 26,005 | ||||||||
| Grant revenue | 431 | 107 | 432 | 162 | ||||||||||||
| Total revenue | 10,533 | 13,160 | 33,684 | 26,167 | ||||||||||||
| Operating costs and expenses | ||||||||||||||||
| Cost of revenue | 10,877 | 15,409 | 32,706 | 37,435 | ||||||||||||
| Research and development | 70,007 | 40,836 | 171,744 | 111,716 | ||||||||||||
| General and administrative | 29,199 | 19,488 | 80,364 | 61,761 | ||||||||||||
| Total operating costs and expenses | 110,083 | 75,733 | 284,814 | 210,912 | ||||||||||||
| Loss from operations | (99,550 | ) | (62,573 | ) | (251,130 | ) | (184,745 | ) | ||||||||
| Other income, net | 6,533 | 2,128 | 16,060 | 2,761 | ||||||||||||
| Net loss | $ | (93,017 | ) | $ | (60,445 | ) | $ | (235,070 | ) | $ | (181,984 | ) | ||||
| Per share data | ||||||||||||||||
| Net loss per share of Class A, B and Exchangeable common stock, basic and diluted | $ | (0.43 | ) | $ | (0.35 | ) | $ | (1.16 | ) | $ | (1.06 | ) | ||||
| Weighted-average shares (Class A, B and Exchangeable) outstanding, basic and diluted | 214,327,186 | 173,435,970 | 203,090,637 | 172,122,974 | ||||||||||||
Consolidated Balance Sheets
| Condensed Consolidated Balance Sheets (unaudited) | ||||||||
| (in thousands) | ||||||||
| 2023 | 2022 | |||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 387,322 | $ | 549,912 | ||||
| Restricted cash | 2,256 | 1,280 | ||||||
| Other receivables | 3,164 | 2,753 | ||||||
| Other current assets | 17,780 | 15,869 | ||||||
| Total current assets | 410,522 | 569,814 | ||||||
| Restricted cash, non-current | 7,629 | 7,920 | ||||||
| Property and equipment, net | 86,248 | 88,192 | ||||||
| Operating lease right-of-use assets | 34,062 | 33,255 | ||||||
| Intangible assets, net | 39,459 | 1,306 | ||||||
| 52,750 | 801 | |||||||
| Other assets, non-current | 155 | - | ||||||
| Total assets | $ | 630,825 | $ | 701,288 | ||||
| Liabilities and stockholders’ equity | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | 4,265 | $ | 4,586 | ||||
| Accrued expenses and other liabilities | 39,806 | 32,904 | ||||||
| Unearned revenue | 43,997 | 56,726 | ||||||
| Notes payable | 695 | 97 | ||||||
| Operating lease liabilities | 5,355 | 5,952 | ||||||
| Total current liabilities | 94,118 | 100,265 | ||||||
| Unearned revenue, non-current | 51,383 | 70,261 | ||||||
| Notes payable, non-current | 1,126 | 536 | ||||||
| Operating lease liabilities, non-current | 44,300 | 44,420 | ||||||
| Deferred tax liabilities | 1,931 | - | ||||||
| Total liabilities | 192,858 | 215,482 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ equity | ||||||||
| Common stock (Class A, B and Exchangeable) | 2 | 2 | ||||||
| Additional paid-in capital | 1,312,591 | 1,125,360 | ||||||
| Accumulated deficit | (874,626 | ) | (639,556 | ) | ||||
| Total stockholder's equity | 437,967 | 485,806 | ||||||
| Total liabilities and stockholders’ equity | $ | 630,825 | $ | 701,288 | ||||
Forward-Looking Statements
This document contains information that includes or is based upon "forward-looking statements" within the meaning of the Securities Litigation Reform Act of 1995, including, without limitation, those regarding the outcomes and benefits expected from access to the real-world multimodal data held at
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/816be0b8-7538-44d3-ae77-526a0f0a4ae0
https://www.globenewswire.com/NewsRoom/AttachmentNg/f6a98a74-af5f-4d00-84fd-99f6738146f3

Source: Recursion Pharmaceuticals