Recursion Provides Business Updates and Reports Third Quarter 2022 Financial Results
- Initiated our Phase 2 clinical trial for the potential treatment of familial adenomatous polyposis (FAP)
- Initiated our Phase 1 clinical trial for the potential treatment of Clostridium difficile colitis
- Nominated a new clinical program in AXIN1/APC mutant cancers with an initial focus on hepatocellular carcinoma and ovarian cancer, for which a Phase 2 clinical trial is being planned
- Raised gross proceeds of approximately $150 million in a private placement offering
SALT LAKE CITY, Nov. 8, 2022 /PRNewswire/ -- Recursion (Nasdaq: RXRX), the clinical-stage biotechnology company industrializing drug discovery by decoding biology, today reported business updates and financial results for its third quarter ending September 30, 2022.
"We are excited to have initiated four clinical trials in the past three quarters," said Chris Gibson, Ph.D., Co-Founder & CEO at Recursion. "In addition, our first clinical stage program discovered using our mapping and navigating approach to biology was nominated as a clinical stage program, with a Phase 2 clinical trial being planned now. We believe that our consistency in advancing our internal pipeline and transformational partnerships coupled with our willingness to continuously evolve our platform to more completely map and navigate biology and chemistry highlight Recursion as a leader within technology-enabled drug discovery."
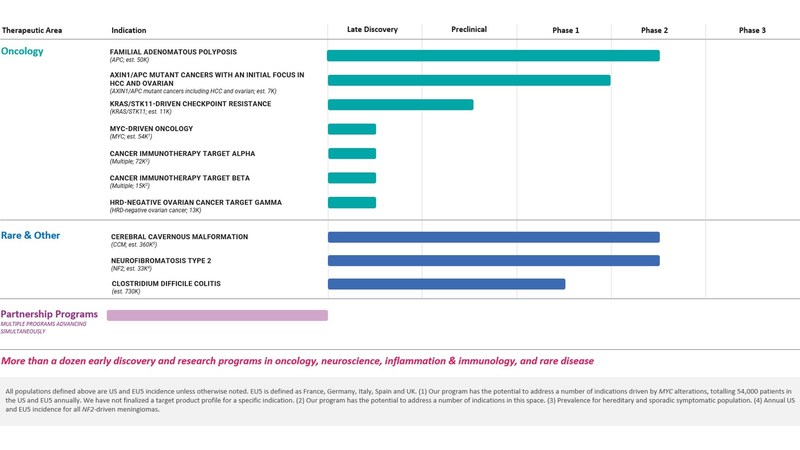
Summary of Business Highlights
- Internal Pipeline
- Cerebral Cavernous Malformation (CCM) (REC-994): In March 2022, we announced the initiation of our Phase 2 SYCAMORE clinical trial, which is a double-blind, placebo-controlled safety, tolerability and exploratory efficacy study of this drug candidate in 60 participants with CCM. At this time, we continue to actively enroll participants.
- Neurofibromatosis Type 2 (NF2) (REC-2282): In June 2022, we announced the initiation of our Phase 2/3 POPLAR clinical trial, which is a parallel group, two stage, randomized, multicenter study of this drug candidate in approximately 90 participants with progressive NF2-mutated meningiomas. At this time, we continue to actively enroll participants.
- Familial Adenomatous Polyposis (FAP) (REC-4881): In September 2022, we announced the initiation of our Phase 2 TUPELO clinical trial, which is a multicenter, randomized, double-blind, placebo-controlled two-part clinical trial to evaluate efficacy, safety, and pharmacokinetics of REC-4881 in patients with FAP.
- AXIN1/APC Mutant Cancers (REC-4881): In October 2022, we announced the nomination of REC-4881 for the potential treatment of AXIN1/APC mutant cancers with an initial focus on hepatocellular carcinoma and ovarian cancer. We have prioritized resources to accelerate planning to initiate a Phase 2 trial. The advancement of this program highlights our intent to focus our internal pipeline on oncology and oncology-like opportunities.
- Clostridium difficile Colitis (REC-3964): In September 2022, we announced the initiation of our Phase 1 clinical trial, which is a first-in-human protocol evaluating single and multiple doses of REC-3964 in healthy volunteers and will assess the safety, tolerability and pharmacokinetic profile of REC-3964.
- GM2 Gangliosidosis (REC-3599): Due to the advancement of our program in AXIN1/APC mutant cancers and the increasing number of oncology programs moving towards the clinic, we deprioritized our GM2 gangliosidosis program and redirected resources. We will make efforts to work with patient foundations to transfer relevant scientific knowledge.
- Transformational Collaborations
- We continue to advance efforts to potentially discover new therapeutics with our strategic partners in the areas of fibrotic disease (Bayer) as well as neuroscience and a single indication in gastrointestinal oncology (Roche and Genentech).
- Recursion OS
- Transcriptomics and Industrialized Validation: We continue to build out our scaled transcriptomics platform which has now been adopted into the research operating plans of the majority of Recursion's active programs in order to drive validation, lead selection, and optimization. We are developing an end-to-end industrialized validation process in order to translate phenomic and transcriptomic insights from our maps of biology and chemistry.
- InVivomics and Digital Tolerability: Digital tolerability is a novel in vivo method for analytical dose selection and interpretation prior to initiating efficacy studies. By the end of the year, we are planning to have 100% of new chemical entities evaluated using digital tolerability before starting any long-term efficacy studies in animals. Furthermore, we continue to increase the dimensionality of digital biomarker signals measured in our preclinical in vivo studies.
- Chemical Technology and Machine Learning: We have completed the design of the remaining core component modules of our automated chemical microsynthesis platform. We envision advanced machine learning approaches as guiding experiment design and drug candidate selection while exploring new ways of building maps of biology and chemistry in order to improve our ability to predict treatments and understand causal mechanisms. Likewise, in the third quarter, we began an initiative in molecular modeling to use predictive and generative methods to drive chemistry optimization.
- Additional Corporate Updates
- Private Placement Offering: On October 27, 2022, we completed a private placement of common stock, raising gross proceeds of approximately $150.3 million, before deducting placement agent fees and other expenses.
- ESG Reporting: In August 2022, we announced receiving a Prime Rating for ESG performance from the industry-renowned Institutional Shareholder Services (ISS). A Prime Rating is awarded to companies with ESG performance above a sector-specific threshold and is assessed by ISS using an "absolute best in class" methodology.
Third Quarter 2022 Financial Results
- Cash Position: Cash and cash equivalents were $454.6 million as of September 30, 2022, which excludes proceeds from the above private placement offering.
- Revenue: Total revenue, consisting primarily of revenue from collaborative agreements, was $13.2 million for the third quarter of 2022, compared to $2.5 million for the third quarter of 2021. The increase was due to revenue recognized from our Roche-Genentech collaboration.
- Research and Development Expenses: Research and development expenses were $40.8 million for the third quarter of 2022, compared to $33.2 million for the third quarter of 2021. The increase in research and development expenses was due to increased clinical costs as studies progressed.
- General and Administrative Expenses: General and administrative expenses were $19.5 million for the third quarter of 2022, compared to $15.7 million for the third quarter of 2021. The increase in general and administrative expenses was due to the growth in size of the company's operations, including an increase in salaries and wages of $4.0 million and other administrative costs associated with operating a public company.
- Net Loss: Net loss was $60.4 million for the third quarter of 2022, compared to a net loss of $47.4 million for the third quarter of 2021.
About Recursion
Recursion is the clinical-stage biotechnology company industrializing drug discovery by decoding biology. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously expands one of the world's largest proprietary biological and chemical datasets. Recursion leverages sophisticated machine-learning algorithms to distill from its dataset a collection of trillions of searchable relationships across biology and chemistry unconstrained by human bias. By commanding massive experimental scale — up to millions of wet lab experiments weekly — and massive computational scale — owning and operating one of the most powerful supercomputers in the world, Recursion is uniting technology, biology and chemistry to advance the future of medicine.
Recursion is headquartered in Salt Lake City, where it is a founding member of BioHive, the Utah life sciences industry collective. Recursion also has offices in Toronto, Montreal and the San Francisco Bay Area. Learn more at www.Recursion.com, or connect on Twitter and LinkedIn.
Media Contact
Media@Recursion.com
Investor Contact
Investor@Recursion.com
Consolidated Statements of Operations
|
Recursion Pharmaceuticals, Inc. |
||||||
|
Condensed Consolidated Statements of Operations (unaudited) |
||||||
|
(in thousands, except share and per share amounts) |
||||||
|
Three months ended |
Nine months ended |
|||||
|
September 30, |
September 30, |
|||||
|
Revenue |
2022 |
2021 |
2022 |
2021 |
||
|
Operating revenue |
$ 13,053 |
$ 2,500 |
$ 26,005 |
$ 7,500 |
||
|
Grant revenue |
107 |
34 |
162 |
145 |
||
|
Total revenue |
13,160 |
2,534 |
26,167 |
7,645 |
||
|
Operating costs and expenses |
||||||
|
Cost of revenue |
15,409 |
- |
37,435 |
- |
||
|
Research and development |
40,836 |
33,246 |
111,716 |
86,979 |
||
|
General and administrative |
19,488 |
15,690 |
61,761 |
38,481 |
||
|
Total operating costs and expenses |
75,733 |
48,936 |
210,912 |
125,460 |
||
|
Loss from operations |
(62,573) |
(46,402) |
(184,745) |
(117,815) |
||
|
Other income (loss), net |
2,128 |
(1,026) |
2,761 |
(3,731) |
||
|
Net loss |
$ (60,445) |
$ (47,428) |
$ (181,984) |
$ (121,546) |
||
|
Per share data |
||||||
|
Net loss per share of Class A and B common stock, basic and diluted |
$ (0.35) |
$ (0.28) |
$ (1.06) |
$ (1.10) |
||
|
Weighted-average shares (Class A and B) outstanding, basic and diluted |
173,435,970 |
168,533,550 |
172,122,974 |
110,513,231 |
||
Consolidated Balance Sheets
|
Recursion Pharmaceuticals, Inc. |
|||
|
Condensed Consolidated Balance Sheets (unaudited) |
|||
|
(in thousands) |
|||
|
September 30, |
December 31, |
||
|
2022 |
2021 |
||
|
Assets |
|||
|
Current assets |
|||
|
Cash and cash equivalents |
$ 454,646 |
$ 285,116 |
|
|
Restricted cash |
2,090 |
1,552 |
|
|
Accounts receivable |
- |
34 |
|
|
Other receivables |
11,635 |
9,056 |
|
|
Investments |
- |
231,446 |
|
|
Other current assets |
13,247 |
7,514 |
|
|
Total current assets |
481,618 |
534,718 |
|
|
Restricted cash, non-current |
8,154 |
8,681 |
|
|
Property and equipment, net |
85,777 |
64,725 |
|
|
Operating lease right-of-use assets |
33,726 |
- |
|
|
Intangible assets, net |
1,457 |
1,385 |
|
|
Goodwill |
801 |
801 |
|
|
Other non-current assets |
- |
35 |
|
|
Total assets |
$ 611,533 |
$ 610,345 |
|
|
Liabilities and stockholders' equity |
|||
|
Current liabilities |
|||
|
Accounts payable |
$ 3,890 |
$ 2,819 |
|
|
Accrued expenses and other liabilities |
26,757 |
32,333 |
|
|
Unearned revenue |
46,753 |
10,000 |
|
|
Notes payable |
95 |
90 |
|
|
Operating lease liabilities |
5,541 |
- |
|
|
Lease incentive obligation |
- |
1,416 |
|
|
Total current liabilities |
83,036 |
46,658 |
|
|
Deferred rent |
- |
4,110 |
|
|
Unearned revenue, non-current |
93,909 |
6,667 |
|
|
Notes payable, non-current |
561 |
633 |
|
|
Operating lease liabilities, non-current |
45,993 |
- |
|
|
Lease incentive obligation, non-current |
- |
9,339 |
|
|
Total liabilities |
223,499 |
67,407 |
|
|
Commitments and contingencies |
|||
|
Stockholders' equity |
|||
|
Common stock (Class A and B) |
2 |
2 |
|
|
Additional paid-in capital |
970,096 |
943,142 |
|
|
Accumulated deficit |
(582,064) |
(400,080) |
|
|
Accumulated other comprehensive loss |
- |
(126) |
|
|
Total stockholder's equity |
388,034 |
542,938 |
|
|
Total liabilities and stockholders' equity |
$ 611,533 |
$ 610,345 |
|
Forward-Looking Statements
This document contains information that includes or is based upon "forward-looking statements" within the meaning of the Securities Litigation Reform Act of 1995, including, without limitation, those regarding early and late stage discovery, preclinical, and clinical programs; licenses and collaborations; prospective products and their potential future indications and market opportunities; Recursion OS and other technologies; business and financial plans and performance; and all other statements that are not historical facts. Forward-looking statements may or may not include identifying words such as "plan," "will," "expect," "anticipate," "intend," "believe," "potential," "continue," and similar terms. These statements are subject to known or unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements, including but not limited to: challenges inherent in pharmaceutical research and development, including the timing and results of preclinical and clinical programs, where the risk of failure is high and failure can occur at any stage prior to or after regulatory approval due to lack of sufficient efficacy, safety considerations, or other factors; our ability to leverage and enhance our drug discovery platform; our ability to obtain financing for development activities and other corporate purposes; the success of our collaboration activities; our ability to obtain regulatory approval of, and ultimately commercialize, drug candidates; our ability to obtain, maintain, and enforce intellectual property protections; cyberattacks or other disruptions to our technology systems; our ability to attract, motivate, and retain key employees and manage our growth; inflation and other macroeconomic issues; and other risks and uncertainties such as those described under the heading "Risk Factors" in our filings with the U.S. Securities and Exchange Commission, including our most recent Quarterly Report on Form 10-Q and our Annual Report on Form 10-K. All forward-looking statements are based on management's current estimates, projections, and assumptions, and Recursion undertakes no obligation to correct or update any such statements, whether as a result of new information, future developments, or otherwise, except to the extent required by applicable law.
SOURCE Recursion